1 Chemical Ecology Unit, Department of Biological Safety, National Institute for Agro-Environmental Sciences, 3-1-3 Kannondai, Tsukuba Science City, Ibaraki 305-8604, Japan, Email yfujii@affrc.go.jp
We have developed a new bioassay called Dish Pack Method for analysing volatile allelochemicals. In this method, leaves of plants were put into one of the holes in a 6-well-multi-dish. One filter paper and 0.7ml of distilled water were added into the other 5 holes, and 7 seeds of lettuce were put on the filter paper. Each side of the multi-dish was sealed and put in incubator. The growth of test plants was measured after 4 days. It is possible to analyze the internal volatile gas collected from the hole with gas-tight-syringe through the septum set up on the holes. By using this method, it was found that Spider flower (Cleome spinosa) contained strong volatile allelochemical. Methyl isothiocyanate (MITC) was identified in Spider flower by GC-MS. This compound completely inhibited the germination and growth of lettuce at 18 ppm (v/v). MITC was much released from injured leaves, and less from injured roots. MITC has already been registered as synthetic pesticides, but MITC was found to be a natural product in Spider flower, and it might be possible to use this plant as natural nematocide to replace Methyl Bromide.
Key Words
Dish Pack Method, volatile chemical, allelochemical, isothiocyanate, spider flower, Cleome spinosa
Introduction
One of the practical applications of allelopathy for the weed control is the use of ground cover plants with allelopathic activity. Cover plants have the characteristic of being short and of rapid growth to cover the ground surface. If they contain allelopathic compounds, they are expected to strongly compete with weeds. In cases in which their flowers or leaves are beautiful, they could also be useful for the amendment of landscape. Application of these cover plants with allelopathic activity for weed control could be a good farming method for sustainable agriculture which could result to the reduction of the amount used for commercial chemicals.
Consequently, we began to screen the allelopathic activity of cover pants by using bioassays. One is the “Pant Box Method”, and the other is the “Sandwich Method”, that we have already developed. In the first method, test plants are planted in agar medium in nearby lettuce seeds, and then the allelopathic activity of exudates from roots are determined. In the latter method, test leaves are dug into agar medium and lettuce seeds are planted on it, and the allelopathic activity of leachates from leaves are determined. Another route of action is evaporation from leaves, but a bioassay has not been developed yet, so we tried to develop new bioassay for volatile allelochemicals from plants.
Methods
Test plants were cultivated for about one month by sand or soil culture, supplying water containing the nutrient solution in greenhouses. The receiver plant used for bioassay was lettuce (Great Lakes 366), since it is highly sensitive to inhibition by allelochemicals. Standards of volatile compounds used in this experiment were commercial products. Used multi-dishes are made in Nunc Company (6 holes, diameter of holes; 3.5 cm).
Used gas chromatograph-mass spectrometer (GC-MS) was model QP-5000 made in Shimadzu Company. Capillary column for GC-MS was HR-Thermon600T (Shin-etsu Chemical Company). The column was 25m long and its inside diameter was 0.25 mm. Pressure of Helium gas as carrier was 37 kPa/min. Injection temperature was 250°. The column was produced an increase in temperature at the rate of 4°/min from 50° to 110°.

Fig 1. Method of Bioassay for Volatile Allelochemical
* Numbers Indicate Distance between the Hole of Test Plants and each Hole.
- Put 2g of cut leaves into one of holes in multi-dish with 6 holes.
- Put filter paper and 0.7ml of distilled water into another 5 holes.
- Seal each side of multi-dish and put it in incubator under 25°, dark condition.
- Check on lettuce growth after 3 days.
- Analyze the internal volatile gas collect from the hole with gas tight syringe
We checked 35 test species mainly of cover plants. Used multi-dishes with 6 holes were same with those used in Sandwich method, except that the area of the cover corresponding to each holes were drilled in the center with a drill. Silicon septums were fitted into the drilled holes. 2 g of fresh leaves were cut by scissors and placed into one of the holes in the multi-dishes. Filter paper and 0.7ml of distilled water were put into the other 5 holes, and 7 seeds of lettuce were put on the filter paper. Each side of the multi-dishes were sealed by cellotape and packed in aluminum foil, then placed in incubator under 25°. Radicle and hypocotyl lengths of lettuce were checked after 4 days. As just described, we could check volatile compounds activities in non-contact system. Speed of diffusion and intensity of activity of volatile compounds were estimated based on the relation of the distance between test plants and lettuce seeds.
The internal volatile gas was analysed with time by GC-MS. The gas (0.5ml) was collected from the hole with a gas tight syringe through the septums set up on the holes. To identify the volatile compounds in test plants, we then used glass bottles (100ml) with holes drilled in the center of their top covers as test instruments. 2g of ground test plants were put in it, then 15 min. after, internal gas (0.5ml) was collected from the bottles with a gas tight syringe and analysed by GC-MS. Additionally volatile standard compounds were tested with the same method.
Results
The screening results for allelopathic cover plants by Dish-pack Method, Plant Box Method and Sandwich Method were shown in Table1. In the case of Plant Box Method and Sandwich Method, allelopathic activities were high in legume. At the same time in the case of Dish-pack Method and Sandwich Method, allelopathic activities of Cleome were the highest of all test plants. Volatile compounds from these test plants were analyzed by GC-MS. From legume, carbonyl compounds (trans-2-Hexenal, et al.) were mainly identified. From oxeye and labiate, monoterpenoids (α-Pinene, Limonene, Myrcene et al.) were identified as the main compounds. And from Cleome, MITC (mustard oil) was identified.
In another test by Dish-pack Method, MITC completely inhibited the germination and growth of lettuce at 18 ppm (v/v). In the same method, leaf aldehyde (trans-2-Hexenal) inhibited growth of lettuce radicle 64% at 21ppm. Inhibitory activity by leaf alcohols (cis-3-Henenol et al.), monoterpenoids (Myrcene, Limonene, Pinene et al.) are not strong. We analysed the concentration of MITC in Cleome. MITC concentration increased in injured leaves, but less from injured roots. MITC was also released in the germination process. Growth inhibitory activity in Dish Pack could be explained by MITC concentration contained in each part of Cleome.
Table 1. Assessment of AUelopathic Activity of Plant Volatile Compounds by Dish-pack Method
Allelopathic Activity*1 (Kind of Bioassay) |
|||||||
Rank |
Scientific Name |
Family |
Dish-pack |
Sandwich*3 |
Plant Box*3 |
Main Volatile Compounds | |
Radicle |
Hypoocotyl |
Radicle |
Radicle |
||||
1 |
Cleome spinosa |
Capparidaceae |
0 |
0 |
0 |
5 7 |
Methyl isothiocyanate |
2 |
Papaver rhoeas |
Papaveraceae |
1 6 |
2 0 |
3 9 |
1 0 |
2-Hexenal |
3 |
Pueraria thunbergiana |
Legurninosae |
2 2 |
3 1 |
6 5 |
2 0 |
2-Hexenal, Hexanal, 4-Pentenal |
4 |
Hibiscus cannabinus |
Malvaceae |
3 1 |
4 6 |
2-Hexenal, 3-Hexenal | ||
5 |
Solidago a1tissima |
Compositae |
3 2 |
3 9 |
6 5 |
7 9 |
α-Pinene, Limonene, Myrcene, Ocimene |
6 |
Vicia vi1losa |
Leguminosae |
3 4 |
4 2 |
1 5 |
3 0 |
2-Hexenal |
7 |
Ficus carica |
Moraceae |
3 4 |
4 2 |
2-Hexenal, 4-Pentenal | ||
8 |
Rosmarinus officinalis |
Labiatae |
3 6 |
2 7 |
9 4 |
α-Pinene, Camphor, Cineole | |
9 |
Crotalaria agatiflora |
Leguminosae |
3 9 |
5 1 |
3 6 |
2 3 |
2-Hexenal, trans-3-Hexenol |
10 |
Hieradum aurantiacum |
Compositae |
3 9 |
4 0 |
8 1 |
||
11 |
Artemisia princips |
Compositae |
4 1 |
4 0 |
8 5 |
p-Pinene, Cineol, 2-Octenal | |
12 |
Pleioblastus |
Gramineae |
4 2 |
5 2 |
4 7 |
trans-3-Hexenol | |
13 |
Vinca major |
Apocinaceae |
4 5 |
5 0 |
3 4 |
cis-3-Hexenyl acetate, trans-3-Hexenol | |
14 |
Robinia pseudo-acacia |
Legurninosae |
5 1 |
5 3 |
3 0 |
1 0 |
2-Hexenal, cis-2-Hexenol |
15 |
Ipomoea aquatica |
Convolvulaceae |
5 4 |
5 8 |
2 2 |
9 8 |
2-Hexenal, 4-Pentenal |
16 |
Silene armeria |
Caryophyllaceae |
5 6 |
6 0 |
2 3 |
4 2 |
2-Hexenal |
17 |
Mucuna pruriens |
Legurninosae |
6 0 |
5 5 |
1 0 |
1 1 |
Hexanal, 2-Hexenal, 3-Hexenal |
18 |
Fagopyrum esculentum |
Polygonaceae |
6 6 |
5 1 |
1 9 |
7 4 |
2-Hexenal, 3-Hexenal |
19 |
Arctotheca calendula |
Composltae |
6 6 |
9 0 |
6 8 |
β-Pinene, 2-Hexenal | |
20 |
Phlox subulata |
Polemoniaceae |
6 8 |
6 6 |
2 3 |
2 9 |
Limonene |
21 |
Phacelia tanacetafolia |
Hydrophyllaceae |
7 1 |
8 0 |
2 6 |
3 2 |
Myrcene, Limonene,2-Hexenal |
22 |
Potentilla verna |
Rosaceae |
7 1 |
6 0 |
6 9 |
2 3 |
|
23 |
Thymus serphyllum |
Labiatae |
7 4 |
7 1 |
4 4 |
Terpinen, Cymene, Isocaryophyllene | |
24 |
Oxa1is articu1ata |
Oxandaceae |
7 5 |
7 8 |
2 3 |
2 8 |
3-Hexen-1-ol acetate |
25 |
Chamomilla nobi1is |
Composltae |
7 9 |
7 5 |
8 3 |
6 0 |
Ocimene, Cyclopropanecamoxylic acid |
26 |
Festuca myuros |
Gramineae |
8 2 |
5 6 |
2 7 |
2 7 |
cis-3-Hexenyl acetate |
27 |
Lampranthu spectabi1is s |
Ficoidaceae |
8 3 |
7 2 |
1 6 |
||
28 |
Coreopsis tinctoria |
Compositae |
8 3 |
7 9 |
8 1 |
9 3 |
Limonene, α -Phellandrene, α -P1nene |
29 |
Sedum sarmentosum |
Crassulaceae |
8 5 |
7 7 |
3 2 |
5 8 |
|
30 |
Mentha pulegium |
Labiatae |
8 7 |
9 2 |
5 0 |
6 6 |
Pulegone, Myrcene, Limonene |
31 |
Zoyosia |
Gramineae |
8 9 |
7 9 |
|||
32 |
Cymbopogon citratus |
Gramineae |
9 2 |
7 5 |
Myrcene, Citral | ||
33 |
Houttuynia cordata |
Saururaceae |
9 3 |
7 5 |
7 4 |
Myrcene, β -P1nene, Ocimene, Limonene | |
34 |
Lycoris radidata |
Amaryllidaceae |
9 4 |
8 7 |
9 4 |
6 5 |
2-Hexenal |
35 |
Ocimum basi1icum |
Labiatae |
9 7 |
8 7 |
8 3 |
8 3 |
Linalol, Cineole |
Eucalyptus citriodora |
Myrtaceae |
0 |
6-0ctenal, β-Pinene, Oclmene, Citral | ||||
Festuca |
Gramineae |
2 5 |
2 3 |
cls-3-Hexenyl acetate | |||
1 Allelopathic activity measured by dish-pack test (new bioassay or volatile compound). All data are compared to the control and 0 means complete inhibition.
2 Radicle(% ), Hypocotyl (%) -radicle or hypocotyl growth of lettuce compared to the control (no test plant).
3 The data or allelopathic activity measured by sandwich test and plant box test are quote from National Institute of Agro- Environmental Science.
Discussion
Bioassay for Allelopathy is important for finding new allelochemicals. Dish-pack Method is a newly developed method for the analysis of volatile allelochemicals. Usually volatile compounds from leaves are diffused by wind and usually difficult to be concentrated enough to have potent inhibitory activity in the field. We have considered that volatile compounds slightly effect interaction between plant and plant except for wood or vegetation environment. But in case of cover plants or their fallen leaves thickly covering the ground surface, it is possible that volatile compounds could reach enough effective concentration to inhibit the germination and growth of other plants. In this report, we found strong volatile allelochemical (MITC) in Cleome. From the point of view of generation mechanism, MITC in Cleome were substances related to aryl isothiocyanate from Japanese horse-radish. We estimate MITC was made from precursor by enzyme reaction. Glycoside as precursor was hydrolysed to MITC by hydrolase in the cells when the plants were injured or germinated. This enzyme was not deactivated even at 60°. Physiological meaning of MITC generation was unknown. It is possible that MITC protect plants from enemies in case of injury or germination.
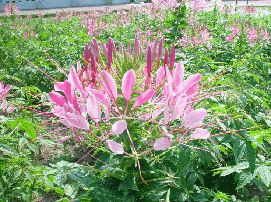
Fig 2. Flower of Spider flower (Cleome spinosa)

Fig 3 Methyl isothiocyanate
Incidentally, Cleome belongs to Capparidaceae, and Japanese horse-radish belongs to Brassicaceae. They are closely related because they both belong to the same order Capparales. Aryl isothiocyanate is known by its antibacterial activity and effect of keeping off insects. “Wasabi”, a Japanese horse-radish, also contain aryl isothiocyanate, and used as spice as a mean of preventing food poisoning, especially in case of “Sashimi” (row fish) and “Sushi”.
Cleome is an ornamen tal flower that has originated in South America. It is perennial in tropical zone, but annual in temperate zone such as Japan, because it could not survive the winter. In Japan, annually during summer, it comes out with splendid pink and white colour in turn. Flowering period is from May to September.
MITC has already registered as chemical synthetic pesticides. MITC is a safety compound for human and environment, because safety test was already carried out for pesticide registration. It is believed that residual toxicity of MITC is low, because of its volatile characteristic. However, effects on other living organisms must be assessed first in order not to damage the ecosystem when introducing new plant containing allelopathic compound in agricultural field. One of the remaining problems is that test plants are injured (unusual condition) in this method, so further improvements are required. And field tests are required as well to confirm the effects of MITC derived from Cleome. In these tests, to find the method wherein the volatile compounds could act effectively is important. Furthermore, allelopathic activities of MITC against fungi and nematode are expected to be assayed. If field tests prove successful, weed control by Cleome could contribute to sustainable agriculture.
References
Fujii Y, Parvez SS, Parvez MM, Ohmae S, Iida O (2003). Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biology and Management 3, 233-241.
Fujii Y, Shibuya T, Nakatani K, Itani T, Hiradate S and Parvez M M (2004). Assessment method for allelopathic effect from leaf litter leachates. Weed Biology and Management 4, 19-23.
Matsuyama M, Hiradate, S Shimozawa, H. Nakatani, K and Fujii Y (2000). Allelopathy of Spider flower (Cleome spinosa) and identification of methyl isothiocyanate as a allelopathic compound. Weed Research, Japan 45 (S), 78-79.
Matsuyama M, Hiradate S, Nakatani K and FujiiY (2000). Developments of new bioassay and analysis method for volatile allelochemicals. Weed Research, Japan 45 (S), 80-81.





