Spatial pattern and mulching effect of Anastatica hierochuntica L. on structure and function of some desert plants
Botany Department, Faculty of Science, Cairo University, Giza 12613, Egypt.
E-mail: akhegazy2202@hotmail.com
A mulching green house experiment was conducted to study the allelopathic effect of the desert annual Anastatica hierochuntica L. (Brassicaceae) on four test species viz., Diplotaxis acris (Forssk.) Boiss., Plantago phaeostoma Boiss. & Helder., Trichodesma africanum (L.) R. Br., and Farsetia aegyptia Turra. The spatial pattern effect of A. hierochuntica L., within and outside its patches, on its allelopathic potential is discussed. The patch was differentiated into outer and core zone microhabitats. The degree of clumping i.e. intraspecific aggregation and the density and phytomass of A. hierochuntica decreased from the core to the outer zone microhabitat of the patch. In the outside patch microhabitat, random distribution was observed at all scales with lower density and phytomass. Consequently, the allelopathic potential of A. hierochuntica decreased from the core zone microhabitat of the patch to the outside patch microhabitat and the importance value of the associate species increased.
Mulching caused lower photosynthetic efficiency and increased hydraulic conductance and leaf specific conductance in treated plants of the four test species. The results demonstrated general reduction in the reproductive attributes in plants raised in mulched soils. The germination tests of seeds produced under the allelopathy treatment showed an inhibitory effect on D. acris and a stimulatory effect on P. phaeostoma and T. africanum, compared to the control treatment.
The spatial pattern of Anastatica hierochuntica affects its allelopathic potential on annual and perennial desert plants. The anatomical peculiarities, reproductive attributes, seed fecundity and dispersion pattern were positively affected.
Key Words
allelopathy, population dispersion, anatomical peculiarities, reproductive attributes, seed offspring.
Introduction
The study species (Anastatica hierochuntica L., Brassicaceae) is a lignified desert annual. Populations of the species, once established in a new site, persist for several years as dry skeletons. Seed release occurs in situ by repeated curling and uncurling of the dry branches due to the hygrochastic nature. The species grows in runnel microhabitats or in depressions forming patches of different sizes. Within a patch, the species shows zones that differed in the spatial pattern, density and total phytomass.
Field observations of a particular species which tended to be surrounded by a bare zone, or have few associate species, as well as its ability to suppress other species as indicated from reduction of size, density, foliage and other growth characters, provoked the investigation of the allelopathic potential of the species (Chou 1999, Kelsey and Everett 1995, Hegazy and El-Tantawy 2002). Moreover, the spatial pattern of species may control to some extent the type and density of neighbors (Stoll and Prati 2001). Desert plants have been found to affect through allelopathy the patterning of vegetation in their immediate vicinity (Mahall and Callaway 1992, Hegazy 1997).
The following questions are raised and need to be elucidated: (1) What is the relationship between the spatial pattern of A. hierochuntica on the distribution and performance of associate species?; (2) May allelopathy be a cause?; and (3) Does the allelopathic effect extend to the seed progeny of treated plants?
Methods
The allelopathic potential of the source species Anastatica hierochuntica L. was tested against four test species. Two of the test species, namely Diplotaxis acris (Forssk.) Boiss. (Brassicaceae) and Plantago phaeostoma Boiss. & Helder. (Plantaginaceae) are annuals and were observed to grow within and outside A. hierochuntica patches, i. e., positively associated with A. hierochuntica. The other two species, namely Trichodesma africanum (L.) R. Br. (Boraginaceae) and Farsetia aegyptia Turra. (Brassicaceae), are perennials and were negatively associated with A. hierochuntica and generally grow outside its patches. Spatial pattern analysis of Anastatica hierochuntica was performed in the plant’s natural habitat associated with the study of the importance value of the test species in different zones of this patch habitat type. A green house experiment was conducted to study the mulch effect on the test species.
Spatial pattern analysis
Ripley’s K-function (Ripley 1976, Haase 2002) was used to study the spatial pattern of A. hierochuntica populations. Ripley’s K-function [K(d)] is a function of the mean number of neighbours over all individuals at different scales. The L-function [L(d)] is used for simpler interpretation by normalizing the K-function to obtain a benchmark of zero (Bessag 1977).
Experiment design, growth conditions and harvest
The experiment was conducted in the open greenhouse. Seeds of the four test species were sown in plastic pots (25 cm height and 18 cm diameter) filled with sandy soil collected from the same habitats occupied by A. hierochuntica in Wadi Hagoul. To minimize the possible root interaction or the pot effect, after seedling establishment, plants were thinned where three individuals left per pot. Two sets of pots were used for each species. In the first set, representing control, seeds were covered by a thin layer (0.5cm) of soil. In the second set, representing the treatment, ground A. hierochuntica dead skeletons (15 g per pot) were mulched on the surface of the sown seeds. Pots were irrigated regularly every 5-7 days with adjusted equal amounts of water to avoid leaching. The experiment time extended for 125 days in the case of the two annual species and 275 days for the two perennial species. The total number of flowers, fruits and seeds was recorded for control and treated plants. Five replicates were used for each reading.
Anatomical study
Anatomical measurements of the seedling petiole and leaf parameters of control and treated plants were performed using a light microscope equipped with an ocular micrometer. The investigated parameters are the thickness of the palisade and spongy tissues in the leaf lamina cross-sections and the density and diameter of xylem conduits in the petiole cross-sections. Ratios of the thickness of the palisade to spongy tissues were used to estimate the photosynthetic efficiency. Theoretical hydraulic conductance (Kh) of the petiole was estimated from anatomical measurements using the Hangen-Poiseuille law (Gibson 1984, Fahmy 1997). The theoretical hydraulic conductance was considered to be a direct estimate of the efficiency of mechanical rise of water. For adjustment of the petiole hydraulic conductance according to the leaf area supplied, the hydraulic conductance was divided by the distal leaf area giving the leaf specific conductance (LSC; Tyree et al. 1983, Fahmy 1997).
Seed germination
Seeds obtained from the test plants raised in the greenhouse experiment were tested for germination. Fifty seeds were used per Petri dish (9 cm diameter). Seeds were allowed to germinate on filter paper (Wattman no. 1) supplied with 5 ml deionized water and incubated at 15 / 25 ºC night / day temperature. The experiment was replicated five times and extended for 15 days.
Results
Spatial pattern analysis and species coexistence
Clumped distribution of A. hierochuntica individuals was observed in the core and outer zones microhabitats of the patch (Figure 1 a and c). The scale of clumping decreased from 10 cm in the core zone to 7 cm in the outer zone. Similarly, the degree of clumping i. e. L(d) values decreased from 8.21 in the core zone to 3.69 in the outer zone. The pattern then shifted to random distribution at larger scales. In the outside patch microhabitat, random distribution of individuals was observed at all scales (Figure 1 e).
The size-class composition together with the contribution of larger size-classes increased from the core zone to the outer zone of the patch (Figure 1 b and d). Higher density of individuals was observed in the core zone especially of the smallest size-class A reaching 32 individual m-2. Concerning the phytomass productivity, however, higher values were recorded in the outer zone reaching 70 gm-2 for size-class C only compared to about 22 gm-2 for size-class A and B collectively in the core zone. Outside the patch lower density of individuals was observed that mostly belong to larger size-classes (Figure 1 f). Comparatively high phytomass productivity was obtained in this case reaching 53 gm-2 for individual belonging to the largest size-class C.

Figure 1. The statistics L (d) in different microhabitats (a, c, e) testing the spatial pattern of Anastatica hierochuntica. Solid lines are the values of the statistics calculated from the data, dotted lines delimit the 95 % confidence regions for the random model. Bars show the density (closed bars) and phytomass (open bars) of A. hierochuntica size-classes in different microhabitats (b, d, f). The volume range of size-classes is: A <1 cm3, B = 1.1 - 5 cm3, C = 5.1 - 20 cm3. Bars topped by the same letter are not significantly different (P < 0.05) within the same series, capital letters show the significance of differences for the density and small letters for the phytomass.
The importance values (IV) of A. hierochuntica and associate species in different microhabitats reflects the dominance of the source species in the core and outer zone microhabitats of the patch (Figure 2). The two annual test species were recorded even in the core zone, however with little IV values that increased towards the outside of the patch. P. phaeostoma has higher values reaching 10.9 and 28.8 as compared to 8.5 and 12 in case of D. acris in the core and outer zone microhabitats respectively. The perennial species were not recorded in the core zone microhabitat. T. africanum was only recorded in the outside patch microhabitat with comparatively lower IV value than F. aegyptia that may be observed in the outer zone microhabitat but with little IV value (3.46).
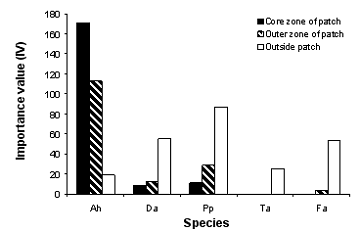
Figure 2. Importance value of Anastatica hierochuntica and associate species in different microhabitats. Ah = Anastatica hierochuntica, Da = Diplotaxis acris, Pp = Plantago phaeostoma, Fa = Farsetia aegyptia, Ta = Trichodesma africanum. IV = relative density + relative cover + relative frequency. The quadrates used are 1 x 1 m2 and replicated five times.
Reproductive attributes
Fewer flowers per individual were recorded in the flowering stage for the treated annual species (Figure 3 a). Similarly in the senescence stage, the number of fruits and seeds per individual (Figure 3 b) in control plants reached 610 and 862 seeds per individual, compared to 355.5 and 436 seed per individual in treated plants of D. acris (P<0.01) and P. phaeostoma (P<0.001), respectively.
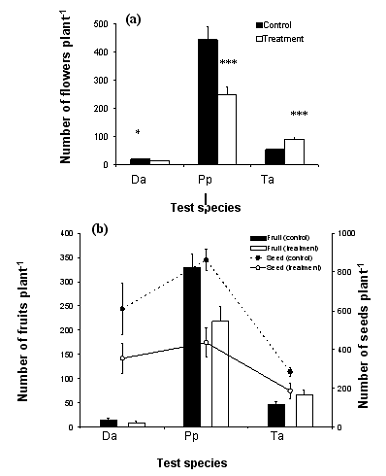
Figure 3. Reproductive attributes of the test species as affected by Anastatica hierochuntica mulching treatment. Da = Diplotaxis acris, Pp = Plantago phaeostoma, and Ta = Trichodesma africanum. (a) Number of flowers per individual plant, and (b) Number of fruits and seeds per individual plant. Vertical bar around the mean is the standard deviation. Significance levels: *P < 0.05, **P < 0.005, ***P < 0.001.
The numbers of flowers and fruits in T. africanum (Figure 3 a and b) were higher in treated plants than in the control. However, the seed output in treated plants was lower than in the control, revealing that some of the fruits contained no seeds. F. aegyptia failed to flower over the experiment time (275 days).
Anatomical features
The ratios of palisade to spongy tissue (Figure 4) were significantly higher in control than in treated plants reaching 5.36 in D. acris control plants. The influence of mulching treatment on the hydraulic conductance of the petiole became more obvious when the leaf area was involved (LSC: leaf specific conductance). Significantly higher LSC in treated than in control plants were obtained in D. acris, P. phaeostoma and T. africanum reaching 9.56 m2 MPa-1 s-1 x 10-20 and 0.82 m2 MPa-1 s-1 x 10-20 in treated and control plants of D. acris (Figure 5).

Figure 4. Ratio of palisade to spongy tissue thickness of leaf lamina in of control and treated seedlings of the test species. Da = Diplotaxis acris, Pp = Plantago phaeostoma, Ta = Trichodesma africanum, and Fa = Farsetia aegyptia. *P<0.05, **P<0.01, ***P<0.001.
![]()

Figure 5. Leaf specific conductance in control and treated seedlings of the test species. Da = Diplotaxis acris, Pp = Plantago phaeostoma, Ta = Trichodesma africanum, and Fa = Farsetia aegyptia. *P<0.05, **P<0.01, ***P<0.001.
Seed fecundity
Treated plants of P. phaeostoma and T. africanum showed significantly lower germination percent than the control (Figure 6). There was no germination of seeds obtained from treated T. africanum plants. The seeds obtained from treated D. acris plants showed a significantly higher germination (35.67 %) compared to 1.81 % for control plants. The dry weight of offspring seeds attained lower values under the mulching treatment for P. phaeostoma and T. africanum. Alternatively, dry weight of seeds obtained from treated D. acris plants was not significantly different from that of the control.

Figure 6. Germination of seed offspring and dry weight of 100 seeds of the test species as affected by Anastatica hierochuntica mulching treatment. Da = Diplotaxis acris, Pp = Plantago phoeostoma and Ta = Trichodesma africanum. Vertical bar around the mean is the standard deviation.
Discussion
The spatial arrangement of plants in a community can be an important determinant of species coexistence and biodiversity (Stoll and Prati 2001). Several plant populations in arid regions were observed to be often monospecific with poor associates e.g. Ammi majus (Friedman et al. 1982), Alhagi graecorum (El-Khatib 1999), Glossonema edule and Anastatica hierochuntica (Hegazy 1997, Hegazy et al. 1990). One hypothesis for this characteristic pattern is the release of toxic allelochemicals which have the potential to regulate the establishment and growth of associate species. In the case of A. hierochuntica, its allelopathic effect was shown on the test species of this study. As intraspecific aggregation and density of individuals increased i. e. from the outer to the core zone of the patch, the amount of soluble allelochemicals increased resulting in higher allelopathic potential and a lower importance value of associate species (D. acris, P. phaeostoma, F. aegyptia, and T. africanum). Outside of the patch microhabitats (outside the core and outer zones of the patch), which is characterized by lower density and random distribution of A. hierochuntica individuals, the species has minimum allelopathic potential on growth of associated species.
Anatomical features
The allelopathic potential of A. hierochuntica was manifested on the plant cell anatomical level of the test species. Mulching treatment caused decrease in the ratio of palisade to spongy tissues indicating lower photosynthetic efficiency in treated plants (Ibrahim and Fahmy 1985). The mulching treatment resulted in increased hydraulic conductance and leaf specific conductance in the four test species. The increase in hydraulic is considered as an adaptation by some species to stressful conditions that may include allelopathic interference (Castro-Diez et al. 1998, Nicotra et al. 2002).
Reproductive attributes
The reproductive growth as represented by the number of flowers, fruits and seeds per individual were suppressed in treated annual plants. This suppression in reproductive growth was shown by allelopathically stressed plants as reported by Hegazy et al. (2001). This feature is considered as a plastic response of the stressed plants which enables them to live but with a diminished reproductive growth (Raynal and Bazzaz 1975). Treated T. africanum plants were reproductively inferior as compared to control plants. This was achieved even though treated plants produced a higher number of flowers and fruits per individual than control, but subsequent stages revealed the sterility of most of these fruits.
Seed fecundity
The allelopathic effect of A. hierochuntica on the germinability of seed offspring of the test species showed both inhibitory and stimulatory effects, i. e. species specific. A. hierochuntica mulches caused reduction in the germination of seeds produced by P. phaeostoma and T. africanum, while germination of seeds produced by treated D. acris plants was significantly higher than that of the control plants. Dormancy of seeds provoked by environmental stress was considered as an inherent character having a role in enabling species to survive environmental stresses that endangered plant’s life (Gutterman 1985, Hegazy, 1990). In the present investigation, dormancy may be a result of allelopathic treatment due to incomplete maturation or deficiency of dry matter investment into the seed offspring that was needed for normal seed development.
Dry seed weight decreased under the mulch treatment for test species, even for D. acris seeds which had higher germination percentage under the treatment. Intraspecific variation in seed mass was reported by McWilliams et al. (1968) as an ecotypic differentiation character. Generally, trade-off exists between seed number and seed size (Smith and Fretwell 1974), but in the present work, treated plants produced fewer seeds with lower dry weight than the control.
Conclusions
Anastatica hierochuntica tended to prevent or decrease the opportunity of other species to invade its patch populations by exerting an allelopathic stress on invaders. This allelopathic potential is characterized by suppressing the vegetative and reproductive attributes of invaders. The allelopathic impact of the species decreased from the core zone microhabitat of the patch to the outside patch microhabitat as the intraspecific aggregation and the density and phytomass of the species decreased.
In the present investigation, annual species were more sensitive to A. hierochuntica allelopathic effect than perennial species. Variation of anatomical and reproductive attributes was apparent. Decrease in fecundity of seed progeny was observed in some cases. All of the study species were affected by the allelopathic interference of A. hierochuntica mulches. The treatment may exert both inhibitory and activator effects on progeny seed germination.
References
Bessag JE (1977). Comments on Ripley’s paper. Journal of the Royal Statistical Society, B 39, 193-195.
Castro-Diez P, Puyravaud JP, Cornelissen JHC and Villar-Salvador P (1998). Stem anatomy and relative growth rate of woody plant species and types. Oecologia 116, 57-66.
Chou CH (1999). Frontiers of allelopathy in sustainable agriculture: experiences from Taiwan. In: Chou CH, Waller GR and Reinhardt (Eds.); Biodiversity and Allelopathy: From Organism to Ecosystems. Academia Sinica, Taipei, Taiwan, pp. 247-261.
El-Khatib AA (1999). An ecological overview on the allelopathy of water hyacinths (Eichornia crassipes), a strategy for weed control. In: F. A. Macias, J. C. J. Galindo, J. M. G. Molinillo and H. G. Culter (eds.); Recent Advances in Allelopathy. Vol. I. A Science for the Future, Cadiz University press, Spain, pp. 471-478.
Fahmy GM (1997). Leaf anatomy and its relation to the ecophysiology of some non-succulent desert plants from Egypt. Journal of Arid Environments 36, 499-525.
Friedman J, Rushkin E and Waller GR (1982). Highly potent germination inhibitors in aqueous eluate of fruits of Bishop’s weed (Ammi majus L.) and avoidance of autoinhibition. Journal of Chemical Ecology 8, 55-65.
Gibson AC, Calkin HW and Nobel PS (1984). Xylem anatomy, water flow, and hydraulic conductance in the fern Cyrtomium falcatum. American Journal of Botany 7, 564-574.
Gutterman Y (1985). Flowering, seed development, and the influence during seed maturation on seed germination of annual weeds. In: S. O. Duke (ed); Weed Physiology. Vol. 1. Reproduction and Ecophysiology. CRC Press, Inc., Boca Raton, Fla., pp. 1-25.
Haase P (2002). Spatial Point Pattern Analysis (SPPA), computer program version 2.0.
Hegazy AK (1990). Growth, phenology, competition and conservation of two desert hygrochastic annuals raised under different watering regimes. Journal of Arid Environment 19, 85-94.
Hegazy AK (1997). Allelopathic effect of Glossonema edule in Qatar. Allelopathy Journal 4, 133-138.
Hegazy AK, Mansour KS and Abdel-Hady NF (1990). Allelopathic and autotoxic effects of Anastatica hierochuntica L. Journal of Chemical Ecology 16, 2183-2193.
Hegazy AK, Amer WM and Khedr AA (2001). Allelopathic effect of Nymphaea lotus L. on growth and yield of cultivated rice around Lake Manzala (Nile Delta). Hydrobiologia 464, 133-142.
Hegazy AK and El-Tantawy H (2002). Significance of allelopathy in conservation ecology of Acacia tortilis in South Sinai, Egypt. Third World Congress on Allelopathy:Challenge for the New Millinnium. Tsukuba, Japan. 26-30 August 2002.
Ibrahim, AA and Fahmy GM (1985). The significance of anatomical characters in the water economy of some desert plants. Annals of Agricultural Science, Moshtohor 23, 145-162.
Kelsey RG & Everett RL (1995). Allelopathy. In:D. J. Bedunah and R. E. Sosebee (eds.); Wild Land Plants: Physiological Ecology and Developmental Morphology. Society for Range Management, USA, pp. 479-549.
Mahall BE and Callaway RM (1992). Root communication mechanisms and intercommunity distributions of two Mojave desert shrubs. Ecology 73, 2145-2151.
McWilliams EL, Landers RQ and Mahlstede JP (1968). Variation in seed weight and germination in populations of Amaranthus retroflexus L. Ecology 49, 296-296.
Nicotra AB, Babicka N and Westoby M (2002). Seedling root anatomy and morphology: an examination of ecological differentiation with rainfall using phylogenetically independent contrasts. Oecologia 130, 136-145.
Raynal DJ and Bazzaz FA (1975). Interference of winter annuals with Ambrosia artemisifolia in early successional fields. Journal of Ecology 56, 37-49.
Ripley BD (1976). The second-order analysis of stationary processes. Journal of Applied Probability 13, 255-266.
Schade JD, Sponseller R, Collins SL and Stiles A. (2003). The influence of Prosopis canopies on understorey vegetation: effect of landscape position. Journal of Vegetation Science 14, 743-750.
Smith CC and Fretwell SD (1974). The optimal balance between size and number of offspring. The American Naturalist 108, 499-506.
Stoll P and Prati D (2001). Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 82, 319-327.
Tyree MT, Graham MED, Cooper KE and Bazos LJ (1983). The hydraulic architecture of Thuja occidentalis. Canadian Journal of Botany 61, 2105-2111.





