School of Agriculture, Riverina-Murray Institute of Higher Education. Wagga Wagga 2650
Introduction
One of the principal objectives for including a legume in a farming rotation is to improve the reserves of nitrogen (N) in the soil. Once soil N levels have been raised, the stored N can be utilised by a period of cropping.
The challenge confronting a farmer is to convert this N reserve into high yielding, high quality (protein) crops as efficiently as possible. This means ensuring that sufficient N is available to meet the yield objectives, whilst minimising the losses of N from the paddock, other than in the grain. The following discussion does not consider the use of fertiliser N except where it influences uptake of soil N.
The Pathway - Soil To Protein
Figure 1 gives a first indication of the key steps involved in the transfer of soil N to grain protein.
Figure 1. Diagrammatic representation of the nitrogen transfer system.

The diagram emphasises the importance of each of the following:
1. The quantity of reserve soil N
In a legume/wheat rotation, this soil N reserve should have increased where a vigorous legume was grown before the wheat crop. As a guide to the importance of reserve soil N, Cooke et al. (1985) showed that grain yield increased by about 1 t/ha for every 1 t/ha of reserve soil N.
The kilograms of N per hectare in the grain closely correlated with grain yield so, generally, grain N yield increased with reserve soil N.
The reserve soil N is not immediately available for plant uptake as it is predominantly in an organic form (>95% in most soils). The quantities of organic N in topsoils (0-10 cm) range from 0.5 t/ha in sandier soils in dry regions to 2 t/ha in clay soils. The range of 1-1.5 t/ha is common in local soils. In the Red Earths, as much N again occurs in the next 20 cm of soil.
2. The organic N comprising the reserve soil N has to be converted to plant available N (as nitrate mainly but to a lesser extent ammonium).
This conversion occurs as a result of microorganisms decomposing organic matter in soil to get energy for their growth. Where the organic matter is high in N, the N in excess of their needs is released into the soil in available forms. The process is called mineralisation.
Organic N is not a uniform material. At least three broad fractions of organic matter are recognised. These are important as different fractions can be decomposed at different rates.
(a) Recently returned and fresh plant residues decompose in a matter of months thus providing little reserve N for more than one wheat crop beyond pasture.
(b) More resistant partly decomposed plant residues are broken down over a 3 to 5 year period. These are the materials which provide most N for crops but make up less than 10% of the organic soil N.
(c) Old humified organic matter is the most abundant fraction but it decomposes very slowly and only makes a small contribution to release of plant available N.
It is important to realise that the process is BIOLOGICAL so that soil conditions such as aeration, temperature, pH and moisture will influence microbial activity and thus nitrate formation. As an example, the rate of mineralisation is slow in spring until surface soil temperatures rise over 10oC (Figure 2). Thus cold spring conditions accentuate N deficiency in crops dependent on N from mineralisation.
Figure 2. Pattern of crop N uptake through two growing seasons.

3. Loss of plant available nitrate
Before wheat can take up nitrate, it may be lost from the root zone.
The nitrate may be leached down through the profile of permeable soils during rainfall period in average years in the Wagga district on Red Earths. The nitrate is not leached out of the root zone and is therefore available for uptake by the crop during later stages of growth (Storrier, 1965). In years of above average rainfall this will not be the case.
Where soils become waterlogged, the nitrate is transformed into nitrogen gases which cannot be taken up by wheat. This biological process is known as denitrification.
4. Plant uptake of N
About two-thirds of the N taken up is transferred to the grain prior to maturity.
The resultant N content of the grain (mainly in the protein form) is influenced by grain yield. Where the N supply is limiting, increasing grain yield reduces the protein content.
The Principle Of Soil N Management
(Matching Soil Supply with Crop demand)
Because nitrate can be lost from the soil, thus reducing the supply to the crop, the management of soil N depends on ensuring:
(i) that sufficient nitrate is in the soil to meet plant demands at each stage of the growth; BUT
(ii) that excess nitrate does not build up such that leaching or denitrification losses result BEFORE the wheat crop can utilise it.
What, then, is a typical pattern of uptake of N by a wheat crop?
Figure 3. Seasonal pattern of temperature (0) and rate of release of available N (^)
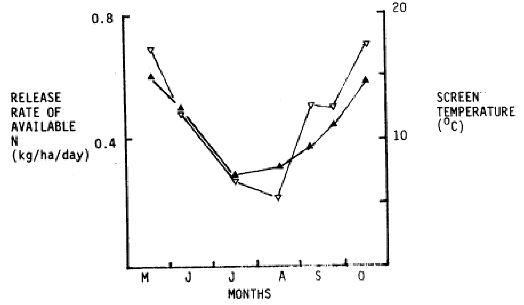
Figure 3 shows that uptake of N is slow over the cooler winter months but accelerates rapidly in August.
To satisfy the high August/September crop demand, two patterns of soil
N release are possible; each with management risks. The alternative soil
N release patterns are
EITHER
(i) during the autumn with nitrate being carried through winter with the risk of loss by leaching or denitrification;
OR
(ii) during the period of growth in spring with the risk that soil temperatures remain low and the biological release of nitrate being restricted.
Farming practice can influence both
• the seasonal pattern of release, and
• the amount of N which is made available.
Management Of Soil N To Protein
The following discussion attempts to isolate the effects of different farm cultural practices on soil nitrogen reserves, the amount and seasonal pattern of plant-available N release, N losses and N uptake.
1. Continuous cropping with wheat
Continuous cropping, either wheat/wheat or wheat/fallow, in southern Australia for periods of 23 to 35 years has normally resulted in a decrease in organic soil N (Clarke and Russell, 1977).
Decreases in organic soil N must occur as the N in the grain is exported from a paddock. Assuming an average N content of grain of 2%, 1, 2 and 4 t/ha grain crops will remove 20, 40 and 80 kg/ha of N. Continuous wheat in the absence of fertiliser N generally decreases grain protein (Dalal and Mayer, 1986; Taylor, pers. comm.).
Organic soil N decreases of less than that removed in grain have been observed, as have soil gains in N under continuous wheat without fallow (Clarke and Russell, 1977). This may be due to fixation of N by organisms other than those associated with legumes (i.e. non-symbiotic N fixation). These organisms depend on straw for an energy supply so that stubble retention systems may favour N input into the soil from this source (Roper, 1987). To this end Parker and Hamilton (1987) demonstrated that total soil N increased with time under direct drilling at 4 of 6 experimental sites but not in traditional cultivation treatments.
2. Cultivation method
Cultivation influences many factors which control yield. Supply of nitrogen is one of these but the discussion which follows attempts to identify the effect of cultivation on nitrogen mineralisation, losses and crop uptake. Stubble management is considered subsequently.
Soil disturbance increases the extent of N mineralisation especially in high clay soils (Craswell and Waring, 1972). As a result, nitrate levels at sowing are normally higher in cultivated than in undisturbed soils. Consequently one of the causes of poor growth of crops established by direct drilling as opposed to cultivation is attributable to low available N levels at sowing (Greenwood et al., 1970; Gates et al., 1981; Mason and Fischer, 1986). However, wheat requires most of its N later in the growing season (Figure 2). Hence the rate of N mineralisation during the late vegetative stage is of prime importance. Field studies have shown that mineralisation rates in spring are similar or higher in direct drilled or reduced tilled compared with cultivated areas (Kohn et al., 1966; Reeve and Ellington, 1974; Stein et al., 1987).
Leaching loss occurs in higher rainfall environments or in exceptionally wet years in the Wagga district (Kohn et al., 1966). Leaching during winter is more likely to be a problem in cultivated soil where the plant-available nitrate is released during the preceding autumn. If leaching rains occur later, generally more water percolates through direct drilled soils (Burch et al., 1986). In soils which show weak structural development this is likely to enhance leaching loss of nitrate. In strongly structured soils, the extra water is likely to travel through large pores reducing leaching of nitrate retained in aggregates.
Loss of nitrate by denitrification has been reported to be higher in uncultivated soils. However, other hydrological features of the profile which promote waterlogging will have a larger effect (for example, an impermeable clay B horizon). However, at Lockhart, NSW, on Red Brown Earth prone to waterlogging, losses appear greater under cultivation.
Because of the differing effects of cultivation technique on mineralisation and losses, the effects on final plant uptake vary with season and soil. For example, N uptake in direct drilled soils have been the same (Stein et al., 1987) or less (Reeve and Ellington, 1974; Gates et al., 1981; Cooke et al., 1985) or even greater (Stein et al., 1987) than in cultivated soil.
The key to all the issues discussed above is how they interact to influence grain yield and protein quality.
On the Red Earth soils of the Wagga Wagga region grain yields from direct drilling are equal or superior to those from cultivation (Rowell et al., 1977; Taylor, A.C., 1986; Cornish and Lymbery, 1986; Pratley and McNeill, 1982).
Grain protein levels have been similar for both treatments on one study (Pratley, pers. comm.). However, where N uptake is the same but grain yield higher in direct drilled compared with cultivation treatments, grain protein levels were lower in direct drilled soil (A. Taylor, pers. comm.).
On soils with poor structure but similar or lower total nitrogen contents such as the Red Brown Earths of Victoria (Reeve and Ellington, 1974; 1969 data when N uptake recorded; Cooke et al., 1985) and Lockhart (Mason and Fischer, 1986) and Yellow Podzolic near Canberra (Gates et al., 1981), grain yields are often lower, by about 10%, from direct drilled compared with crops grown in disturbed soil. Lower grain yields were associated with lower protein contents on Yellow Podzolics at Canberra (Gates et al., 1981) but overall on the Victorian Red Brown Earths grain protein was unaffected by cultivation method (Cooke et al., 1985).
3. Stubble management
(a) Burning
The implications of stubble burning to soil N management have been known for a long time. Hallsworth et al. (1954) found in a survey of farms in northern NSW that burning reduced the soil N reserve (total soil N concentration) (Table 1).
Table 1. Effect of management on soil total N concentration (adapted from Hallsworth et al., 1954).
Management |
Soil total N (%) |
Stubble ploughed in |
0.14 |
Stubble burnt |
0.11 |
Clean fallow |
0.09 |
The proportion of N taken up by a wheat crop and remaining in the stubble averages 25%. Thus in wheat crops yielding 2 and 4 t/ha, this translates into about 13 and 26 kg/ha of N in the stubble respectively. When stubble is burnt, the N is converted into a gaseous form which is not returned to the soil. On local Red Earth soils, most of the loss in soil N can be accounted for in grain N removal and burning of stubble (A.S. Black and J.E. Pratley, unpublished).
(b) Grazing
Where stubble grazing is practised, most of the stubble N consumed is returned to the soil as dung and urine. Under the dry conditions which exist in this district in autumn, between 20 to 80% of N returned in urine is likely to be lost as ammonia gas.
However, Mulholland (1986) estimated that less than 30% of stubble would be consumed in an average year. Losses associated with this intensity of grazing are likely to be small.
(c) Retention
Where stubble is retained and incorporated by cultivation, the soil microorganisms are provided with a high carbon source. This will result in utilisation of plant-available nitrate and conversion to plant- unavailable organic forms (immobilisation). The N is normally not released until the carbon levels from stubble fall. This period appears to be of the order of 3 to 4 months depending on soil moisture and temperature. As an example, Bacon and Osborne (1987) reported that incorporation of stubble three months before sowing, and at sowing, resulted in uptake of 71 and 54 kg N/ha respectively. On a Red Earth at Wagga retention of residue reduced yields by about 10-15% compared with burning (A.C. Taylor, 1986).
Because N uptake was higher in the burnt compared with the stubble retained treatment by a similar proportion to grain yield, protein levels were similar in both treatments.
Straw incorporation may be beneficial in high fertility paddocks as excess nitrate would be immobilised and not prone to leaching and denitrification losses over winter.
When stubble is retained on the surface and not incorporated, as may occur in direct drilling, the stubble is less prone to rapid decomposition. The extent of immobilisation is likely to be less than when stubble is incorporated. For example, Bacon and Osborne (1987) reported N uptake of 73, 61 and 54 kg/ha for burnt-no cultivation, retained on surface-no cultivation and stubble incorporated by cultivation respectively. Similar trends have been observed at Wagga on Red Earths. Again, because grain and N uptake changed by similar proportions, protein content was unaffected (A.C.Taylor, pers. comm.).
4. Application of fertiliser nitrogen
On occasions application of fertiliser N has improved the uptake of soil N. Bacon (1987) demonstrated that where stubble had been left on the surface, uptake of soil N was 56 kg N/ha but when N fertiliser was applied this was increased to 87 kg N/ha. Where stubble was incorporated, burnt and direct drilled or burnt and the soil cultivated, no effect of N fertiliser was observed.
The increased soil N uptake after N fertiliser application may result from a number of causes, for example, enhanced microbial breakdown of soil organic N or improved recovery of available N from soil.
5. Liming
For acid soils, the rate of decomposition of organic matter is normally reduced. Consequently one of the effects of raising the pH through liming is to increase the rate of N mineralisation. This effect has been observed in pastures growing on acid soils (Awad and Edwards, 1977;
Edmeades et al., 1981). The effect is usually only present in the season of lime application.
6. Agricultural chemicals
Agricultural chemicals do influence the activity of soil microorganisms. However, the concentrations of herbicides and pesticides which may be expected to occur in soil at recommended rates of application, do not appear to be sufficient to have a 25% reduction in the rate of mineralisation (Goring and Laskowski, 1982).
Conclusions
1. Endeavour by appropriate management to match release of N from the soil with crop demand.
2. Soil N release (mineralisation) is a biological process. Since seasonal factors such as temperature, moisture and aeration (which are notoriously variable from year to year) influence release, assessment of supply problems may be best left until July/August.
3. Wheat monoculture will normally deplete soil N.
4. Cultivation technique generally alters the pattern of N mineralisation.
5. Stubble retention reduces N uptake and keeps the N in the soil.
6. N fertiliser and lime occasionally provide a period of enhanced N mineralisation.
References
1. Awad, A.S. and Edwards, D.G. (1977). Reversal of adverse effects of heavy ammonium sulphate application on growth and nutrient status of kikuyu pastures. Plant Soil 48, 169-183.
2. Bacon, P.E. (1987). Management strategies to maximise soil N mineralisation under wheat and rice. In ‘Nitrogen cycling in temperate agricultural systems’, Bacon, P.E., Evans, J., Storrier, R.R. and Taylor, A.C. pp.244-SO. (Aust.Soc.Soil Sci.Inc., Riverina Branch, Wagga Wagga).
3. Bacon, P.E. and Osborne, G.J. (1987). Effect of tillage and residues on the N cycle. In ‘Nitrogen cycling in temperate agricultural systems’. Bacon, P.E., Evans, J., Storrier, R.R. and Taylor, A.C. pp.360-379 (Aust.Soc.Soil Sci.Inc., Riverina Branch, Wagga Wagga).
4. Burch, G.J., Mason, I.B., Fischer, R.A. and Moore, 1.9. (1986). Tillage effects on soils: physical and hydraulic responses to direct drilling at Lockhart, NSW. Aust.J.Soil Res. 24, 377-91.
5. Clarke, A.L. and Russell, J.S. (1977). Crop sequential practices. In ‘Soil factors in crop production in a semi-arid environment’, Russell, J.S. and Greacen, E.L. pp. 279-300.
6. Cooke, J.W., Ford, G.W., Dumsday, R.G. and Willott, S.T. (1985). The effect of fallowing practices on the growth and yield of wheat in south-eastern Australia. Aust.J.Exp.Agric. 25, 614-27.
7. Cornish, P.S. and Lymbery, J.R. (1986). The agronomy of direct drilled wheat crops. In Proc. Conf. Recent Advances in Weed and Crop R&idue Management, Pratley, J.E. and Cornish, P.S. pp.6-8 (RMIHE Wagga Wagga).
8. Craswell, E.T. and Waring, S.A. (1972). Effect of grinding on the decomposition of soil organic matter. I. The mineralization of organic nitrogen in relation to soil type. Soil Biol.Biochem. 4, 427-33.
9. Dalal, R.D. and Mayer, R.J. (1986). Long term trends in fertility of soils under continuous cultivation and cereal cropping in southern Queensland. V. Rate of loss of total nitrogen from the soil profile and changes in carbon:nitrogen ratios. Aust.J.Soil Res. 24, 493-504.
10. Edmeades, D.C., Judd, M. and Sarathchandra, S.V. (1981). The effect of lime on nitrogen mineralization as measured by grass growth. Plant Soil 60, 177-186.
11. Gates, T.C., Jones, D.B., Muller, W.J. and Hicks, J.S. (1981). The interaction of nutrients and tillage methods on wheat and weed developipent. Aust.J.Agric. Res. 32, 227-41.
12. Greenwood, E.A.N., Boyd, W.J.R., Whitehead, J.A. and Titmanis, Z.V. (1970). Effect of time, cultivation and urea on nitrogen stress and yield of wheat in a low rainfall area of Western Australia. Aust.J.Exp.Agric.Anim.Husb. 10, 763-767.
13. Goring, C.A.I. and Laskowski, D.A. (1982). The effects of pesticides on nitrogen transformations in soils. In ‘Nitrogen in agricultural soils Stevenson, F.J. pp.689-719 (Agron.Soc.Amer., Crop Sci.Soc.Amer., Soil Sci. Soc.Amer.: Wisconsin).
14. Hallsworth, E.G., Gibbons, F.R. and Lemerle, T.H. (1954). The nutrient status and cultivation practices of the soils of the north-west wheat belt of New South Wales. Aust.J.Agric.Res. 5, 422-447.
15. Kohn, G.D., Storrier, R.R. and Cuthbertson, E.G. (1966). Fallowing and wheat production in southern New South Wales. Aust.J .Exp.Ajric.Anim.Husb. 6, 233-241.
16. Mason, I.B. and Fischer, R.A. (1986). Tillage practices and the growth and yield of wheat in southern New South Wales: Lockhart, in the 450 mm rainfall region. Aust.J.Exp.Agric. 26, 457-68.
17. Mulholland, J.G. (1986). The removal of crop residues by sheep and cattle. In ‘Proc.Conf. Advances in Weed and Crop Residues Management’, Pratley, J.E. and Cornish, P.S. pp.133-4 (RMIHE Wagga Wagga).
18. Parker, I.J. and Hamilton, G.J. (1987). Total nitrogen and organic carbon trends under a range of tillage practices. In ‘Nitrogen cycling in temperate agricultural systems’, Bacon, P.E., Evans, J., Storrier, R.R. and Taylor, A.C. pp.380-389. (Aust.Soc.Soil Sci. Inc., Riverina Branch, Wagga Wagga).
19. Pratley, J.E. and McNeill, A.A. (1982). A comparison of seedbed preparation methods for wheat productions. Proc.2nd.Aust.Agron.Conf., Wagga Wagga, 201.
20. Reeves, T.G. and Ellington, A. (1974). Direct drilling experiments with wheat. Aust.J.Exp.Agric.Anim.Husb. 14, 237-240.
21. Roper, M.M. (1987). N fixation in soil by free-living bacteria utilizing straw as a source of energy. In ‘Nitrogen cycling in temperate agricultural systems’. Bacon, P.E., Evans. J., Storrier, R.R., Taylor, A.C. pp.143-47. (Aust.Soc.Soil Sci.Inc., Riverina Branch, Wagga Wagga).
22. Rowell, D.L., Osborne, G.J., Matthews, P.G., Stonebridge, W.C. and McNeill, A.A. (1977). The effects in a long-term trial of minimum and reduced cultivation on wheat yields. Aust.J.Exp.Agric.Anim.Husb. 17, 802-11.
23. Stein, J.A., Sageman, A.R., Fischer, R.A. and Angus, J.F. (1987). Soil nitrogen supply of wheat in relation to method of cultivation. Soil Tillage Res. 10, 243-258.
24. Storrier, R.R. (1965). The leaching of nitrogen and its uptake by wheat. Aust.J.Exp.Agric.Anim.Husb. 5, 323-328.
25. Taylor, A.C. (1986). Crop responses to rotation, residue management and tillage in a long-term experiment. In ‘Proc.Conf.Recent Advances in Weed and Crop Residue Management’, Pratley, J.E. and Cornish, P.S. pp.21-24 (RMIHE Wagga Wagga)





