The University of Queensland, School of Land and Food Sciences, St Lucia, Qld 4072. www.uq.edu.au/soils/ Email h.so@uq.edu.au
The measurement of exchangeable cations in saline soils is limited by the difficulty in accurately separating soluble cations from exchangeable cations. A method is examined for saline soils in which exchangeable cations are calculated as the total extractable cations minus the concentration of soil solution (soluble) cations. In addition, a further two standard methods were investigated, one which assumes the total soil extractable cations are exchangeable, the other utilises a pretreatment to remove soluble salts prior to measurement of the remaining (exchangeable) cations. After equilibration with a range of sodium adsorption ratio (SAR) solutions at various ionic strengths, the exchangeable cation concentrations of two soils (Dermosol and Vertosol) were determined by these methods and compared to known values. The assumption that exchangeable cations can be estimated as the total soil extractable cations, although valid at low ionic strength, resulted in an overestimation of exchangeable Na and Ca concentrations at higher ionic strengths due to the presence of soluble salts. Pretreatment with ethanol and glycerol was found to effectively remove soluble salts thus allowing the accurate measurement of the effective cation exchange capacity (ECEC), however, dilution associated with the pretreatment process decreased concentrations of exchangeable Ca while simultaneously increasing exchangeable Na. Using the proposed method, good correlations were found between known and measured concentrations of exchangeable Na (Dermosol: y=0.873x and Vertosol: y=0.960x) and Ca (Dermosol: y=0.906x, and Vertosol: y=1.05x). Therefore, for soils with an ionic strength of approximately 50 mM (ECse 4 dS m-1) or greater (in which exchangeable cation concentrations are overestimated by assuming the total soil cations are exchangeable), concentrations can be calculated as difference between total extractable cations and soluble cations.
Key Words
Effective cation exchange capacity, exchangeable cations, pretreatment for salts, salinity, soil solution
Introduction
Saline soils are of increasing importance both in Australia and world-wide. In Australia, approximately 2.5 M ha of arable land are affected by dryland salinity, costing A$200 M in lost production annually (Australian State of the Environment Committee 2002). In addition, the soil application of poor quality irrigation water may result in an increase in the soils salinity. Nevertheless, measurement of a soils exchange properties under saline conditions has proven to be difficult.
While the measurement of cation exchange capacity (CEC) in saline soils by the replacement of exchangeable cations by a saturating solution is typically quite accurate (providing the soluble salts are adequately removed prior to measurement), this method does not allow the determination of individual exchangeable cation concentrations but merely a whole soil CEC. The effective cation exchange capacity (ECEC) of a soil (theoretically equal to the CEC) is calculated as the sum of the exchangeable cations. In order to measure the soils exchangeable cation concentrations, accurate partitioning between the exchangeable and soluble forms is required. This is particularly true for saline soils where soluble cations may account for a substantial proportion of the extractable cations.
Richards (1954) proposed that the concentration of exchangeable cations could be determined from a saturated extract by determining the concentration of extractable cations less than the concentration of soluble cations. This method, however, has not been validated against known data, and is only rarely used, with other quicker and simpler methods used in preference. These other commonly used methods for the measurement of exchangeable cations in saline soils are generally of two types. The first type assumes all cations extracted from the soil are exchangeable, making no correction for soluble salts. This failure to adequately account for soluble cations prior to measurement results in an overestimation of exchangeable cations. For non-saline soils, however, this error resulting from the presence of soluble salts is typically small (see Menzies and Bell (1988)). The second type of method is those in which soluble cations are removed from the soil prior to the extraction of the remaining (exchangeable) cations. However, for variable charge soils, the decrease in ionic strength associated with this pretreatment process results in a decrease in CEC (Uehara and Gillman 1981), a release (and loss) of exchangeable cations, and hence an underestimate of ECEC. In addition, for soils containing free minerals (such as gypsiferous or calcareous soils) incomplete removal of the free minerals during pretreatment will result in an overestimation of ECEC due to dissolution of the minerals (and release of cations) into the extractant. Further, dilution of the soil during pretreatment may result in a redistribution of cations, with Ca replacing Na on the exchange.
The objective of the work presented here was to measure exchangeable cation concentrations using one of each of these two types of commonly used methods (exchangeable cations estimated as the extractable cations, and the use of a pretreatment step), and to compare these results with known values. In addition, exchangeable cations concentrations were determined by the subtraction of soluble cations concentrations from the total extractable cations concentrations, and results compared to known values. For all methods, results were obtained for two test soils.
Methods
Two soils, a Dermosol and a Vertosol (Isbell 2002), were collected (Beaudesert, Queensland, Australia) and air-dried (Table 1). The dominant clay minerals present were determined for both soils using X-ray diffraction (XRD) analysis of the <2 Ám fraction (Philips PW1800, 0.05░ 2 theta steps with 3.0 s counting per step, quantitative analysis using SIROQUANT (Sietronics Pty Ltd)). Using NaCl and CaCl2.2H2O at predetermined rates (Table 2), 15 solutions were prepared (comprising five SAR treatments (3, 6, 12, 18, and 24 (mmolc L-1)0.5) at three ionic strengths (10, 50, and 150 mM)). Leaching columns were prepared to allow the equilibration of the two soil types with the 15 solutions, each treatment with two replicates (yielding a total of 60 soils). Solution was leached through each of the soils (approximately 300 g air-dry) until the electrical conductivity (EC) of the leachate was similar to that of the initial equilibrating solution (approximately 10 pore volumes).
Table 1. Major clay minerals of the Dermosol and Vertosol (as determined by quantitative XRD analysis, <2 Ám fraction), and selected properties of their soil solutions
Major clay minerals |
pH |
EC |
Na |
Ca |
Mg |
K | |
dS m-1 |
mM | ||||||
Dermosol |
Montmorillonite (58 %), kaolinite (33%) |
5.95 |
7.79 |
39.8 |
16.9 |
0.16 |
0.57 |
Vertosol |
Montmorillonite (78 %), kaolinite (20 %) |
7.56 |
3.22 |
18.0 |
3.17 |
4.20 |
0.33 |
Table 2. Concentrations of Na (as NaCl) and Ca (as CaCl2.2H2O) used for the preparation of the 15 equilibrating solutions of various sodium adsorption ratios (SARs)
Ionic strength (mM) |
SAR |
Na |
Ca |
10 |
3 |
4.15 |
1.94 |
6 |
6.44 |
1.17 | |
12 |
8.43 |
0.50 | |
18 |
9.14 |
0.26 | |
24 |
9.46 |
0.16 | |
50 |
3 |
10.8 |
13.1 |
6 |
19.1 |
10.3 | |
12 |
30.3 |
6.48 | |
18 |
36.9 |
4.27 | |
24 |
40.9 |
2.94 | |
150 |
3 |
19.6 |
43.4 |
6 |
36.6 |
37.7 | |
12 |
63.7 |
28.6 | |
18 |
83.6 |
21.9 | |
24 |
98.2 |
17.0 |
Determining known exchangeable cation concentrations (method A)
In order to allow accurate comparison of the various methods, following equilibration with various SAR solutions, soil exchangeable cation concentrations were determined by a standard method (Doering et al. 1982; Marsi and Evangelou 1991; Poonia et al. 1984; Rhoades 1967; Sposito et al. 1983). A sub-sample (approximately 10 g) was removed from each of the soils following leaching and oven-dried to determine moisture content. On the basis of this value, approximately 6 g Dermosol and 7 g of Vertosol (calculated for each treatment as 4.00 g air-dry equivalent soil) was removed from each column and placed in a 50 mL tube, with the volume of entrained equilibrating SAR solution in each tube calculated by mass. Total cation concentrations (exchangeable plus entrained cations) were determined by inductively coupled plasma atomic emission spectroscopy (ICPAES) following extraction with 40 mL 0.1 M BaCl2/0.1 M NH4Cl (Gillman et al. 1982). Using the concentration of cations calculated to be in the entrained solution, exchangeable cations were determined by the subtraction of entrained cations from total extractable cations.
Removal of soluble cations (method B)
The effect of pretreatment for soluble salts (as described by Rayment and Higginson (1992)) on soil exchangeable cations was investigated. All remaining soil was removed from the leaching columns and air-dried before a further 4.00 g (air-dry) sub-sample was removed and placed in a 50 mL tube. Using 60 % ethanol and 20 % glycerol, each sample was pretreated for soluble salts as described by Rayment and Higginson (1992). A 1:5 soil:ethanol suspension was prepared, shaken for 30 min, centrifuged, and the supernatant discarded. The process was repeated twice more, firstly with ethanol before finally with glycerol. The quantity of entrained solution in the soil was determined by mass, and the exchangeable cation concentrations determined using 40 mL 0.1 M BaCl2/0.1 M NH4Cl as before.
Extraction of total soil cations (method C)
A method was examined in which soluble salts are not removed from the soil, and all extracted cations are assumed to be exchangeable. Although based on Sumner and Miller (1996), 0.1 M BaCl2/0.1 M NH4Cl was used as the extractant rather than 0.2 M CaCl2/0.125 M CaSO4 in order to allow the determination of exchangeable Ca2+ in addition to Mg2+, K+ and Na+. Another 4.00 g air-dry sub-sample was removed from each soil and placed in a 50 mL tube, with cations extracted using 40 mL 0.1 M BaCl2/0.1 M NH4Cl. Cation concentrations were determined using ICPAES and exchangeable cations calculated as the total extractant concentration.
Measurement of total and soluble cations (method D)
A method was examined for the measurement of exchangeable cations in saline soils where determined as the difference between the soluble and extractable cations (Richards 1954). A further 4.00 g air-dry sub-sample was removed from each soil and placed in a 50 mL tube, and total extractable cation concentrations determined using 40 mL 0.1 M BaCl2/0.1 M NH4Cl. Of the remaining air-dry soil, approximately 125 g was wetted to field capacity using triple de-ionised water and allowed to equilibrate for 48 h in a closed box lined with wet paper towelling to minimise evaporative loss (Menzies and Bell 1988). The soil solution was extracted using centrifuge drainage (Gillman 1976), filtered to 0.22 Ám (Millipore GSWP) and cation concentrations determined using ICPAES. The soil exchangeable cations were then calculated as the difference in concentration between total extractable and soil solution cations.
Using GenStat 6 (GenStat 2002), a two-way analysis of variance (completely randomised design) of the ECEC as calculated from each of the methods was performed for both soils. Comparisons between means were made using Fisher’s protected least significant difference (LSD) test. A linear regression was performed to examine the relationship between the measured and actual exchangeable Na and Ca concentrations for each of the methods and for both soils.
Results and discussion
Using the results obtained from each of the various methods, ECEC was calculated as the sum of exchangeable cations. For ECEC, significant interactions were found between ionic strength and method of ECEC measurement for both the Dermosol (LSD (5 %) = 0.437, p<0.001) and the Vertosol (LSD (5 %) = 1.53), indicating significantly different patterns of response across ionic strength by the measurement methods examined (Figure 1). Ionic strength did not affect the actual ECEC (method A), with no significant differences between values at any ionic strength for either the Dermosol or the Vertosol (Figure 1).

Figure 1. Effect of equilibrating solution ionic strength on the effective cation exchange capacity (ECEC) of (a) the Dermosol, and (b) the Vertosol, measured as the actual ECEC (method A) (●), ECEC following pretreatment for soluble salts (method B) (![]() ), ECEC of air-dry soil calculated using total (soluble and exchangeable) soil cations (method C) (○), and ECEC of air-dry soil calculated as total minus soluble cations (method D) (▼) (results are the arithmetic mean of five sodium adsorption ratio (SAR) solutions and two replicates)
), ECEC of air-dry soil calculated using total (soluble and exchangeable) soil cations (method C) (○), and ECEC of air-dry soil calculated as total minus soluble cations (method D) (▼) (results are the arithmetic mean of five sodium adsorption ratio (SAR) solutions and two replicates)
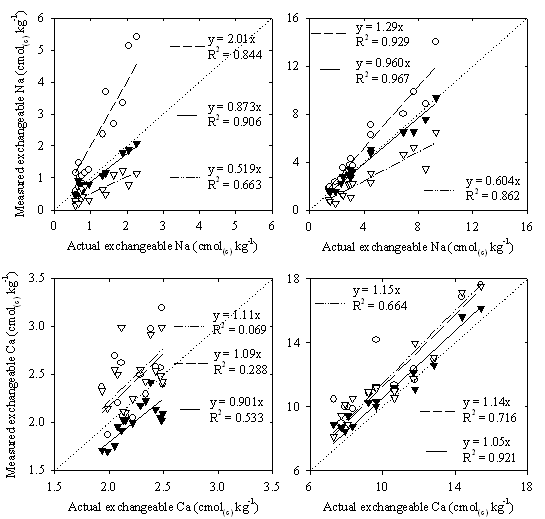
Figure 2. Exchangeable concentrations of Na (top) and Ca (bottom) for the Dermosol (left) and Vertosol (right) (measured following pretreatment for soluble salts (method B) (![]() (– ∙∙ –)), measured as the total (soluble and exchangeable) soil cations (method C) (○ (— —)), and measured as the total minus soluble cations (method D) (▼ (—))) in comparison to actual exchangeable concentrations (method A) (results are the arithmetic mean of two replicates) (dotted line represents y = x)
(– ∙∙ –)), measured as the total (soluble and exchangeable) soil cations (method C) (○ (— —)), and measured as the total minus soluble cations (method D) (▼ (—))) in comparison to actual exchangeable concentrations (method A) (results are the arithmetic mean of two replicates) (dotted line represents y = x)
At low ionic strength of 10 mM there is no significant difference between the actual ECEC and the calculated total soil cations (exchangeable plus soluble) (method C) for either Dermosol or the Vertosol (Figure 1). However, as ionic strength increased, the ECEC calculated from the total soil cations was found to increase significantly with each increase in ionic strength for both soils (Figure 1). This overestimation of ECEC is due to a failure to account for the presence of soluble cations in the soil solution. At low ionic strengths, when only low concentrations of soluble cations were present, the contribution of these soluble cations to the total soil cations was low, and no significant differences were found between results obtained using this method and the actual ECEC. However, as ionic strength increased (and concentrations of soluble salts in the soil solution increased), the failure to account for these soluble cations resulted in elevated ECEC measurements. These high ECECs resulted from high concentrations of both exchangeable Na and exchangeable Ca, with exchangeable Na concentrations 2.0 times higher than actual concentrations in the Dermosol, and 1.3 times higher in the Vertosol, and exchangeable Ca concentrations 1.1 times higher in the Dermosol and 1.1 times higher in the Vertosol (Figure 2).
This method is therefore suitable for the measurement of exchangeable cations in non-saline soils in which the concentration of soluble salts are low. However, as salinity increases, the accuracy of this method decreases (although the percentage error is dependant upon the soil properties (see below)). From the data presented (Figure 1), the measurement of exchangeable cations from the total soil cations is therefore generally only suitable for soils with soil solution ionic strengths less than approximately 50 mM (EC of approximately 4 dS m-1), with errors increasing with increasing ionic strength.
Using this method (method C), the degree to which ECEC (and exchangeable Na and Ca) is overestimated at a given ionic strength is not constant, but dependent upon two factors: (1) the actual ECEC of the soil, and (2) the percentage moisture content of the soil at field capacity. The percentage contribution of soluble cations to the measured ‘exchangeable’ (extractable) cations will decrease as the soils actual ECEC increases. For example, from Figure 1 it can be seen that at the highest ionic strength (150 mM), although the measured ECEC is approximately 4 cmol(c) kg-1 greater than the actual concentration for both the Dermosol and the Vertosol, the relative overestimation using this method is greater in the Dermosol (68 % greater) than the Vertosol (12 % greater) due to the comparatively low ECEC of the Dermosol. In addition, the greater the moisture content of the soil at field capacity (at a particular ionic strength), the greater the quantity of soluble cations present in the soil solution which will measured and attributed to ‘exchangeable’ cations.
Values of ECEC obtained from soil pretreated for soluble salts (method B) were generally similar to actual ECEC values, with significant differences observed between the two methods only at 10 mM in the Dermosol (Figure 1). In addition, for soil pretreated for soluble salts ECEC tended to remain constant across all ionic strengths, although a slight significant difference was found for the Dermosol between ECEC values at 10 and 150 mM (Figure 1). Pretreatment for soluble salts using ethanol and glycerol is therefore an effective method for the removal of soluble salts from the soil solution, with measured values of ECEC measured even in high ionic strength (saline) soils similar to actual ECEC values. However, although the ECEC can be relatively accurately measured following pretreatment for soluble salts, the distribution of exchangeable cations comprising this ECEC was found to be different to the actual composition (Figure 2). Exchangeable Na concentrations were approximately half of that expected for both the Dermosol (y=0.519x) and the Vertosol (y=0.604x), while exchangeable Ca concentrations increased slightly in the Dermosol (y=1.11x) and in the Vertosol (y=1.15x) (Figure 2). This shift in exchangeable cation composition is due dilutional effects, with the dilution of the soil system resulting in an increased preference of the exchange for Ca over Na, thereby increasing exchangeable Ca while decreasing exchangeable Na (Black 1965).
Pretreatment of a soil for soluble salts prior to the extraction of exchangeable cations is recommended if the EC (1:5 soil:water suspension) exceeds 0.3 dS m-1 (Rayment and Higginson 1992). Tucker (1985) reported that pretreatment using ethanol and glycerol effectively removed soluble salts with “minimum disturbance of the exchangeable cations”. However, Gupta et al. (1985) observed that alcohol solutions may alter the degree of solvation of exchangeable cations and the dielectric constant of the solution, thus effecting the double-layer environment of the exchange. The data from this study suggest that while ECEC can be determined from soils pretreated for soluble salts, dilutional effects preclude the use of this method for the accurate measurement of concentrations of the individual exchangeable cations.
Ionic strength did not affect ECEC values calculated as total minus soluble cations (method D), with no significant differences in ECEC between ionic strengths for either soil (Figure 1). Also, no significant differences were found between actual ECEC values and those calculated as total minus soluble cations (method D) at any ionic strength for the Vertosol. For the Dermosol, however, calculated ECEC values were significantly lower than actual values at all three ionic strengths, underestimating ECEC by an average of 14 % (Figure 1). These low ECEC values measured in the Dermosol are attributable to an underestimation in concentrations of both exchangeable Na (y=0.873x) and Ca (y=0.906x) (Figure 2). For the Vertosol, measured concentrations of exchangeable cations correlated well with actual values for both Na (y=0.960x) and Ca (y=1.05x) (Figure 2). For saline soils, concentrations of exchangeable cations can therefore be calculated as the total extractable cations minus soil solution (soluble) cations. The errors observed from this method (Figure 1 and Figure 2) are most likely due to the multiple analysis involved (total soil cations, and soil solution cations), and variability associated with the extraction of soil solution.
Conclusion
In conclusion, in soils with low ionic strength, exchangeable cation concentrations can be estimated from the total soil concentrations (as described by Sumner and Miller (1996)) due to the relatively low concentrations of soluble cations. However, as ionic strength increases, failure to account for soluble salts results in an overestimation of both exchangeable Ca and Na, the magnitude of the error increasing with ionic strength. Pretreatment of a soil using ethanol and glycerol (as described by Rayment and Higginson (1992)) was found to be effective in removing soluble salts, with measured ECECs generally not significantly different to actual values. However, although the ECEC can be accurately measured following pretreatment, the dilution associated with this method resulted in an increase in exchangeable Ca and a decrease in exchangeable Na. Concentrations of exchangeable cations calculated as the total extractable cations minus soil solution (soluble) cations were observed to approximate actual concentrations, even in high ionic strength soils. For soils with a soil solution ionic strength greater than 50 mM (approximately 4 dS m-1), in which exchangeable cations cannot be accurately calculated from the total cations, exchangeable cations can therefore be calculated as the difference between total extractable cations and soil solution cations.
Acknowledgments
The authors acknowledge the assistance of David Bowen and David Appleton with the chemical analyses and soil preparation. Cameron Smeal is also acknowledged for his support and for placing the research in the context of the gelatine industry. This work was conducted as part of an environmental research program funded by Gelita Australia Pty Ltd, and the Australian Research Council (Project Number 2001000896).
References
Australian State of the Environment Committee (2002) 'Australia, State of the Environment 2001.' (CSIRO Publishing: Melbourne)
Black CA (1965) 'Methods of Soil Analysis.' (American Society of Agronomy: Madison, Wis.)
Doering EJ, Willis WO, Merrill SD (1982) Effect of total cation concentration and sodium adsorption ratio on exchangeable sodium ratio. Canadian Journal of Soil Science 62, 177-185.
GenStat (2002) GenStat for Windows. Release 6.1. Sixth Edition. In. (VSN International Ltd., Oxford.)
Gillman GP (1976) 'A Centrifuge Method for Obtaining Soil Solution.' Commonwealth Scientific and Industrial Research Organisation, Brisbane, Australia.
Gillman GP, Skjemstad JO, Bruce RC (1982) 'A Comparison of Methods Used in Queensland for Determining Cation Exchange Properties.' Commonwealth Scientific and Industrial Research Organisation, Brisbane, Queensland.
Gupta RK, Singh CP, Abrol IP (1985) Determining cation exchange capacity and exchangeable sodium in alkali. Soil Science 139, 326-332.
Isbell RF (2002) 'The Australian Soil Classification.' (CSIRO Australia: Collingwood, Victoria)
Marsi M, Evangelou VP (1991) Chemical and physical behavior of two Kentucky soils: I. Sodium-calcium exchange. Journal of Environmental Science and Health Part A Toxic-Hazardous Substances & Environmental Engineering 267, 1147-1176.
Menzies NW, Bell LC (1988) Evaluation of the influence of sample preparation and extraction technique on soil solution composition. Australian Journal of Soil Research 26, 451-464.
Poonia SR, Mehta SC, Pal R (1984) The effects of electrolyte concentrations on calcium sodium exchange equilibria in 2 soil samples of India. Geoderma 32, 63-70.
Rayment GE, Higginson FR (1992) 'Australian Laboratory Handbook of Soil and Water Chemical Methods.' (Inkata Press: Melbourne)
Rhoades JD (1967) Cation exchange reactions of soil and speciment vermiculites. Soil Science Society of America Proceedings 31, 361-365.
Richards LA (1954) 'Diagnosis and Improvement of Saline and Alkali Soils.' (United States Deptartment of Agriculture: Washington)
Sposito G, Holtzclaw KM, Jouany C, Charlet L (1983) Cation selectivity in sodium-calcium, sodium-magnesium, and calcium-magnesium exchange on Wyoming bentonite at 298 K. Soil Science Society of America Journal 47, 917-921.
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In 'Methods of Soil Analysis. Part 3. Chemical Methods'. (Ed. JM Bartels). (SSSA and ASA: Madison, WI)
Tucker BM (1985) Active and exchangeable cations in soils. Australian Journal of Soil Research 23, 195-209.
Uehara G, Gillman G (1981) 'The Mineralogy, Chemistry, and Physics of Tropical Soils with Variable Charge Clays.' (Westview Press: Boulder, Colorado)






